This is one of those questions that we don’t really ask ourselves. We already know that when there is a fire, you need water. We’ve been taught that ever since we were kids, it’s almost like you are born with that kind of information. That is why we don’t really ask ourselves WHY? Why does water put out fire? Well, today we’re going to do just that.
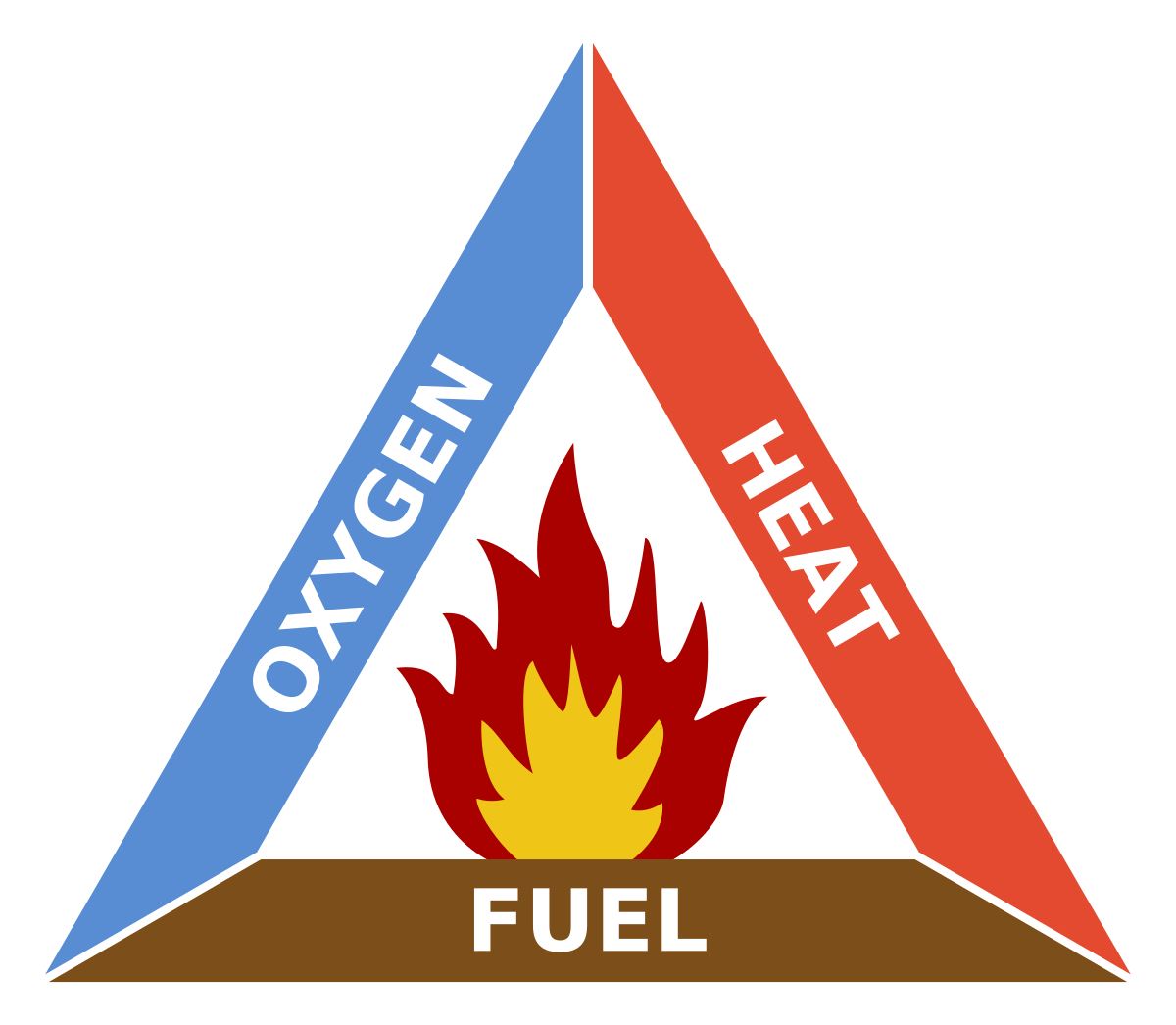
The first thing we need to do is establish what you need to sustain a fire. There are three important elements: fuel, oxygen and heat. So, if you want to put fire out, you need to remove one of these factors. You can’t really remove all of its fuel, because it is already burning and you can’t take away whole houses and stuff like that to deprive fire of its “food”. You can’t really remove oxygen for known reasons, so, you have to take out the heat and water does that. Water removes heat.
 via Wikipedia
via Wikipedia
[the_ad id=”71925″]
Why does water put out fire – explained in detail
Actually, water absorbs heat, due to its evaporation property, at a 540 calories/gram rate. Water, actually, evaporates 7 times faster than it boils. So, that’s what happens. You pour water over the fire and it removes heat. Warm water might be a little bit less efficient for cooling, but not bad at all. If you put warm water over fire, it will work a little bit slower, but, at the same time, warm water creates more vapor which can work as an asphyxiant that pushes away the atmospheric oxygen.
Here’s a great video by NileBlue explaining this
Other ways to put out fires instead of water
Anyways, using water is not always the most effective method to put out fires. In cases of fires that are sustained by liquid fuel, the force of water can disperse the fuel in the air and it doesn’t cool the fire. Moreover, water can actually disperse drops of fuel away from the fire and thus help the fire extend at a faster rate.
- In cases of chemical fires, water can speed up the fire because the fuels can react with water, like in the case of alkali metals. So, we made another weapon against fire. Remember when we were telling you about depriving fire of oxygen? Well, you can’t erase all the oxygen in that area, but you can use a special foam or powder that removes oxygen from where the fire burns. This is the case for carbon-dioxide. This is an oxygen free gas that kills the chemical reaction of fire.
- Also, you can use sand or anything like that because it will reduce the contact zone between what it burns and air, which has oxygen in it, of course.